Molecular DX
TB-LAMP and Malaria-LAMP: Fast, accurate and life-saving
Molecular diagnostics is one of the fastest-growing segments within in vitro diagnostics. It analyzes the genetic material of humans or pathogens to obtain clinical information related to diseases.
Our Molecular Diagnostics solutions are designed to provide high-quality, rapid, and reliable detection of infectious diseases.
Utilizing Loop-mediated Isothermal Amplification (LAMP) technology, our products deliver accurate results with minimal equipment needs, making them ideal for use in low-resource settings.
In cooperation with our partner EIKEN CHEMICALS CO. LTD., HUMAN provides an innovative LAMP testing system for the screening of infections with bacteria belonging to the Mycobacterium Tuberculosis Complex (MTBC) as well as for the Malaria screening (Malaria Pan, Malaria Pf and Malaria Pv) ideal especially in low prevalence areas.

- TB-LAMP used for screening infections caused by bacteria belonging to the Mycobacterium Tuberculosis Complex (MTBC).
- Malaria-LAMP to detect Plasmodium species, Plasmodium ovale, P. vivax, P. malariae, P. knowlesi and P. falciparum.
Loop-mediated isothermal amplification (LAMP)
Advanced molecular detection in 4 easy steps
Loop-mediated isothermal amplification (LAMP) is an accurate, simple, rapid and costeffective molecular method used for pathogen detection. It is characterized by the use of four different primers, which are specifically designed to recognize six distinct regions on the target gene and by a constant temperature during the amplification process. The formed dumbbell-like structures of the DNA provide an increased number of starting points for the DNA synthesis and thus offer a faster multiplication of the target gene.
It enables the amplification at one temperature and the detection of target DNA.The amplified products are detected visually by reading green fluorescence or by the measurement of turbidity in real-time.
Performing LAMP assays is straightforward and requires significantly less technical expertise compared to more complex molecular techniques like polymerase chain reaction (PCR). This allows healthcare workers with basic training to accurately perform the tests.
Its robustness, high sensitivity, and ease of use make LAMP an excellent and nearly failsafe tool for improving diagnostics in remote areas.
Its robustness, high sensitivity, and ease of use make LAMP an excellent and nearly failsafe tool for improving diagnostics in remote areas.
The entire workflow consists of only four major steps: the sample transfer and lysis, the DNA/RNA extraction with the help of the LoopampTM PURE DNA Extraction Kit , the loop-mediated isothermal amplification, and the result reading with the HumaLoop or the HumaTurb system.
1. Easy sample transfer and lysis process
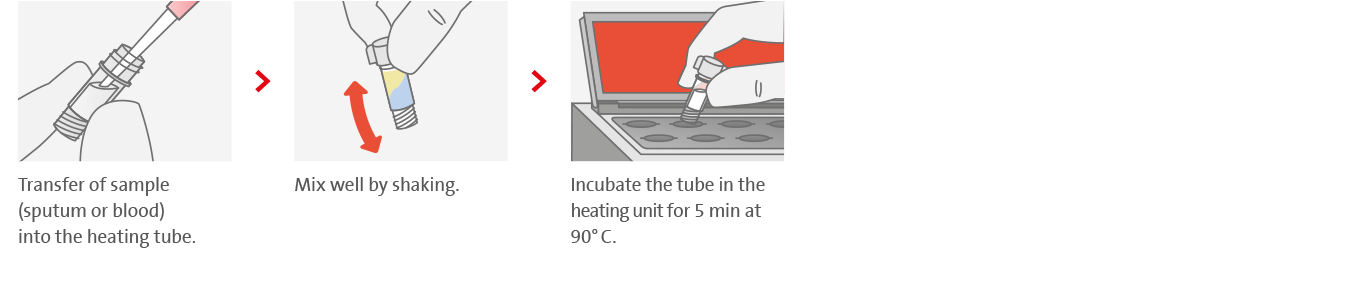
2. Simple and fast DNA extraction
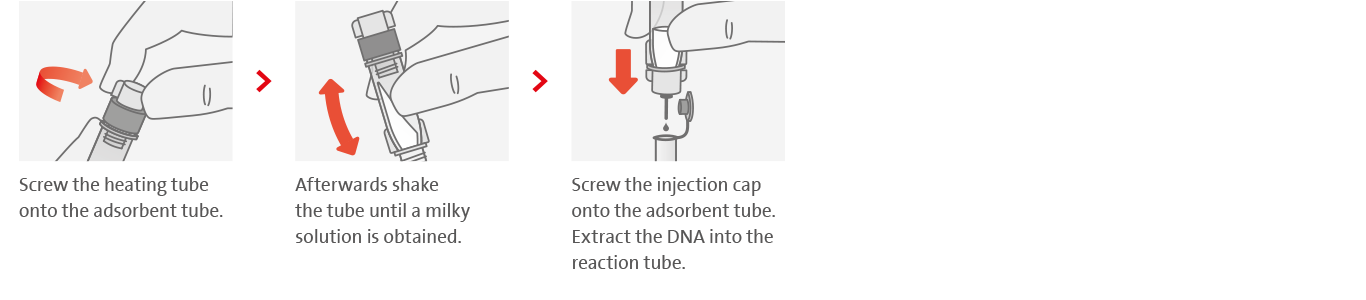
3. Loop-mediated isothermal amplification
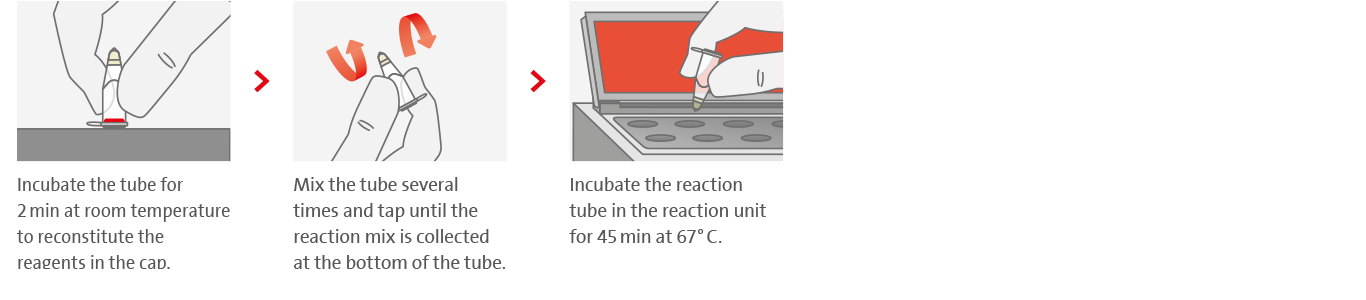
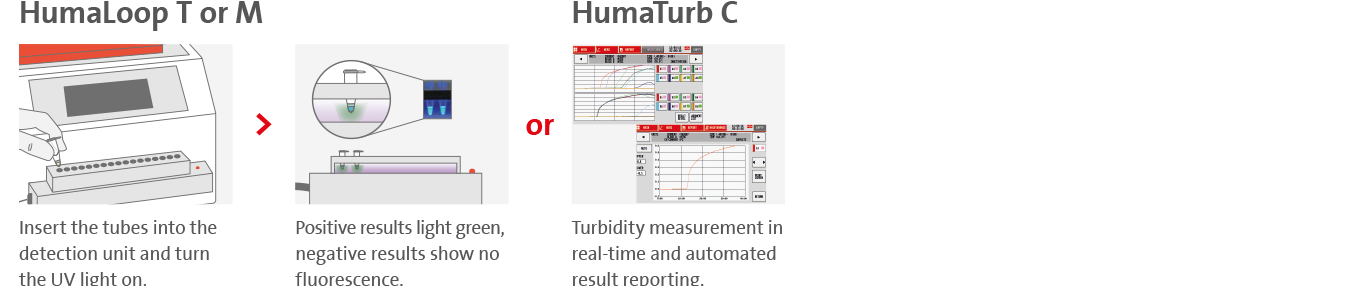
4. Effortless result interpretation
Visually with HumaLoop T and HumaLoop M or by using the HumaTurb turbidimeter
TB-LAMP
Lighting the way to easier, faster, and more accurate Tuberculosis detection
Tuberculosis (TB) remains a major global health concern, with millions of new infections and deaths each year. Accurate and rapid diagnosis is critical for effective treatment and control.
WHO recommends TB-LAMP as an alternative for smear microscopy since 2016. Almost half of all positive TB cases are missed by smear microscopy.
The TB-LAMP (Loop-mediated Isothermal Amplification) test provides a fast, reliable, and affordable molecular method to detect Mycobacterium tuberculosis complex (MTBC) even in resource-limited settings. This cutting-edge technology delivers diagnostic results comparable to standard molecular methods, but with greater simplicity and ease of use.
- High sensitivity and specificity: Accuracy equivalent to traditional PCR
- Rapid detection: Results available within 1–2 hours
- Simple Workflow: Requires minimal training and lab infastructure
- Cost-Effective: TB-LAMP provides a low-cost alternative to other molecular TB detection methods
- Increased accessibility even in remote areas: Portable and independent from mains
For the TB-LAMP solution, additional services are available, such as DataToCare by Savics, a customizable suite of digital applications that streamline laboratory data collection and sharing. It connects national laboratory networks by capturing diagnostic and patient data at the facility level. Another service is SmartSpot Quality, an External Quality Control Program.
DataToCare by Savics for TB-LAMP | Better recording and reporting of tuberculosis cases
New connectivity solution for TB-LAMP helps reduce gap between diagnosis and treatment of TB patients

EQA program for TB-LAMP
EQA program for the TB-LAMP solution established

HUMAN and SmartSpot Quality successfully implemented an External Quality Control Program for TB-LAMP as an ideal solution for applications in remote settings.
From now on it will be possible to verify the functionality of the HumaLoop T, the reagents as well as the laboratory procedures with blinded controls for External Quality Assessment (EQA) and unblinded controls for Verification.
Verification and EQA programs are essential for identifying errors and their causes as early as possible so as to initiate appropriate corrective actions to ensure the accuracy of future testing. By partaking in SmartSpot’s Verification and EQA programs, participants, and the patients they serve, can have the highest possible level of confidence in their results.
The SmartSpot patented technology enables the packaging of inactivated Mycobacterium tuberculosis on a simple spot on a card, which is easily and safely tested like a patient’s clinical specimen. Each card weighs less than 3g, is easy, safe and cost-effective to transport, and is stable for 24 months at ambient temperatures. SmartSpot designs controls for both Verification and EQA.
Verification
involves testing 8 standardized controls on a diagnostic instrument to ensure that it is fit-for-purpose before clinical specimens are to be tested.
EQA
is designed to ensure on-going quality monitoring of diagnostics, from pre-analytical to post-analytical processing. The EQA program consists of 3 test events per year whereby 4 controls are tested per event. As the SmartSpot quality controls are stable for 24 months, all controls that are required for the year are delivered in a single shipment, reducing shipping costs by 67%.
About SmartSpot
Since the onset of widescale molecular testing for TB in 2010, SmartSpot has been developing controls to meet the quality needs of TB molecular laboratories across the globe. SmartSpot Quality is a South African based private company which started in 2010 as a research program at the Department of Molecular Medicine and Haematology (DMMH) and the Centre of Excellence for Biomedical TB Research (CBTBR) at the University of Witwatersrand. Global demand for the SmartSpot controls required scaled up production and as such the University registered SmartSpot Quality (Pty) Limited as a company to execute this role. SmartSpot has been granted an exclusive license to the patented technology and the University departments have continued to improve the technology as well as adapt it to develop controls for additional diseases. In 10 years, SmartSpot has developed a global footprint in 30 countries providing Ministries of Health, Clinical Trial Units and private laboratories with quality control solutions. SmartSpot is the largest commercial supplier of molecular TB controls and provides a companion diagnostic for qualifying the accuracy of molecular diagnostic tests including TB, HIV, SARS-CoV-2, and soon MRSA and HCV.
For more information about SmartSpot, please visit www.SmartSpotQ.com
Two system solutions for various healthcare settings
HumaLoop T
Easy to use LAMP technology for primary and district laboratories
The HumaLoop T is specifically designed as a consolidated platform for sample preparation, amplification, and easy visual result interpretation. The Loopamp™ MTBC Detection Kit enables sensitive and accurate molecular detection of the Mycobacterium tuberculosis complex, offering a valuable alternative to smear microscopy. The elimination of tuberculosis (TB) requires appropriate technology that can be utilized in rural and remote areas.

- Throughput: Up to 16 tests per run or up to 70 samples per day
- Preinstalled settings: Fixed incubation times and temperatures for the Loopamp™ MTBC Detection Kit
- Consolidated processing: Sample preparation, amplification, and detection on a single instrument
- Optimized for remote use: Independent power solution available via solar panel and battery system
- Clear result interpretation: Visual reading of fluorescence signals
- Fast reporting: Results available within 1–2 hours
HumaTurb C+A
Scalable LAMP system for reference and regional laboratories
The HumaTurb system enables real-time detection of turbidity based on magnesium pyrophosphate generated during the amplification process. This system consists of two parts: HumaTurb C and HumaTurb A. HumaTurb C manages the setup and controls the incubation time and temperature, while the amplification occurs in HumaTurb A. Sample lysis is handled by the HumaHeat incubator, which simplifies lysis using Loopamp™ heating tubes and a dedicated heating block.

- Throughput: Up to 96 tests per run if expanded with six HumaTurb A units
- Versatile assays: Multiple Loopamp™ assays can be run simultaneously
- Flexible data transfer: Includes USB connectivity
- Convenient reporting: Built-in printer for automated result generation
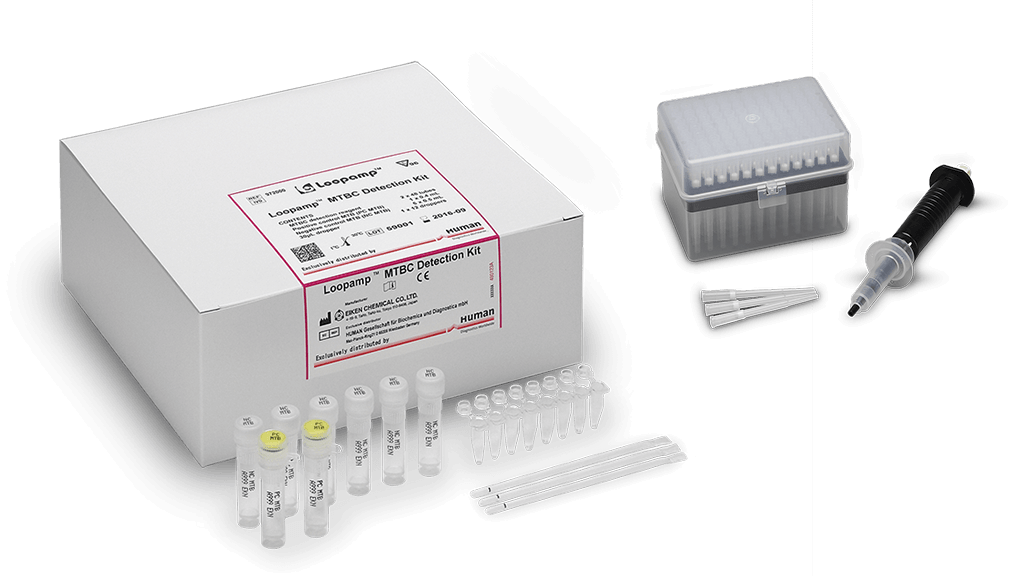
TB-LAMP Loopamp™ Detection Kits
Loopamp™ PURE DNA Extraction Kit
The procedure for Ultra Rapid Extraction (PURE) is a fast and efficient extraction method to obtain highly pure DNA from patient samples. This kit supports up to 90 extractions
Loopamp™MTBC Detection Kit
The Loopamp™ MTBC Detection Kit is a qualitative test for the detection of bacteria belonging to Mycobacterium Tuberculosis Complex (MTBC) in human sputum samples. The kit contains reagents for 96 reactions as well as positive and negative controls. Dried LAMP reagents are reconstituted with extracted DNA solution and incubated for 40 min at 67°C either in HumaLoop T or HumaTurb instruments.
Pipette-60 Set
Pipette to ease the transfer of sputum samples (1 x pipette and 4 x 96 filter tips)
TB-LAMP - It's time for a change
The TB-LAMP workflow allows an easy DNA extraction and preparation of reaction mixes in a few steps with only a minimum of equipment and reagents. Reagent storage and shipment at room temperature as well as the excellent test performance make molecular pathogen testing available in rural areas with limited settings. Only lever to find the missing cases is the access to sensitive and reliable diagnosis. Therefore: TB-LAMP – It’s time for a change!

